forastero
Posts: 458
Joined: Oct. 2011
|
| Quote (JonF @ Nov. 14 2011,15:08) | | Quote (noncarborundum @ Nov. 14 2011,15:23) | | Quote (JonF @ Nov. 14 2011,13:05) | Here, forastero, I'll make it simple for you. We know that, in any radiosotope, the number of atoms that decay per unit time is proportional to the number of atoms present. If there are N radioisotope atoms present and "t" is time, then the change in the number of atoms per unit time, dN/dt, is given by:
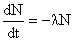
Where lambda is the decay constant.
If we start counting time from whatever point the process started, and there were N0 atoms at that time, the number of atoms present at time t is:
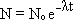
(see Radiometric Dating to see how the second equation is derived from the first).
Now, a moron might say that, since there is exponentiation involved in both this equation and the compound interest equation, then changes in the decay constant lambda will compound. but lambda and time "t" appear in the exponent. So when we solve for "t", the age of the sample:

we see that the relationship between "t" and lambda is hyperbolic, not exponential. A change in lambda results in a inversely proportional change in the calculated age, but if you increase the change in lambda the effect on "t" decreases as the change in lambda increases. A 0.5% change in lambda at t=0 (the best case for you, applying the changed rate throughout the entire process) changes the calculated age of the sample by 0.4975%.
(How we determine N0 is interesting but is outside the scope of this discussion. N and lambda are, of course, measured in the present.)
This equation applies to simple-accumulation methods, such as K-Ar. The more advanced age-diagnostic methods are governed by more complex equations, but the relationship between "t" and lambda is always inversely proportional. For example, the Pb-Pb isochron which is the way we find the total age of the Earth:
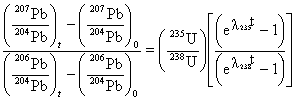
which is not solvable analytically, but can be solved using a computer. Note that "t" appears in the exponent multiplied by lambda, so we know that as in the above example "t" is inversely proportional to lambda. |
The sound you just heard was that of this post going right over forastero's head. |
Of course. He hasn't a prayer.
I don't know of any dating methods, isochron or not, called "U-Pb-He" or "Ne-Ne" or "U-U". There is (U-Th)/He which results in a stable 4He isotope and is not an isochron method. There is cosmogenic exposure dating involving the radioisotope 21Ne, but that's not an isochron method. I suppose there could be U-U disequilibrium dating, but that wouldn't be an isochron method.
The only radioisotope daughter product in his list is U. And it appears that three of the methods in his list are not isochron methods, which is the class of methods under discussion. |
U-U http://www.inqua2011.ch/?a=prog....onid=85
(U-Th)/He http://www.mendeley.com/researc....-ages-9 http://europa.agu.org/?uri=....article
"U-Pb/ He" http://www.icr.org/article....g-world ?
|


















