Tracy P. Hamilton

Posts: 1239
Joined: May 2006
|
| Quote (Henry J @ Sep. 24 2008,11:04) | | Quote (Tracy P. Hamilton @ Sep. 24 2008,09:19) | | Quote (Lou FCD @ Sep. 20 2008,13:18) | 4. Phospate Group
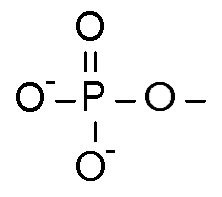
A Phosphate Group is a Phosphorus bonded with two negatively charged Oxygen atoms, one regular Oxygen atom, and double bonded with one Oxygen atom. It's molecular formula is PO<sub>4</sub><sup>2-</sup> or sometimes OPO<sub>3</sub><sup>2-</sup>, to separate the double bonded Oxygen.
Here is where the lecture ended. Although I asked in a later lecture about the odd bondings here that seem to break the rules that we earlier set forth, Doc basically said, "It's complicated, and you don't need to know that for this class, though you'll learn about it in a Chemistry class if you take one." Ok, fair enough. |
Chemical education discourages the use of the double bond in phosphate, and in sulfate groups as well. As you noticed, it violates the octet rule, and does so unnecessarily! There are some molecules where we chemists violate the rule out of necessity, such as having five atoms bonded to P, which requires 5 bonds.
The double bonds in phosphate and sulfate are historical, hence the biologists still use it a lot because that is how they learned it. :O |
So is the proper way to do it to put a - sign on three of the O's and a + sign on the P? |
YES. Don't listen to the old fogey Louis.
What Louis says is correct, though.
Chemists have this resonance idea, but the question is which is more important: a structure that obeys the octet rule but has +1 on P and -1 on each O, or a structure that disobeys the octet rule and has charge 0 on P, and 0 on one O. Is having a positive charge on P so bad, and having negative on O so bad? Answer: no. Louis finds it offensive, apparently. :p
I wonder if Louis would draw BF3 with a double bond or not, and whether there is any pi bonding in it (his justification for drawing P=O in phosphate).
--------------
"Following what I just wrote about fitness, you’re taking refuge in what we see in the world." PaV
"The simple equation F = MA leads to the concept of four-dimensional space." GilDodgen
"We have no brain, I don't, for thinking." Robert Byers
|


















