Lou FCD

Posts: 5455
Joined: Jan. 2006
|

My notes and thoughts from Biology 111, for Monday, September 15, 2008. The entire series can be found here.
The class started with a reminder that the next class period (Wednesday the 17<sup>th</sup>) would be our first exam.
Then there was a short review of Hydrocarbons generally, and carbohydrates specifically, just to get us back to the place where we had left off.
We picked up this lecture with our discussion of Lipids.
2. Lipids
Triglycerides ---> Fats & Oils
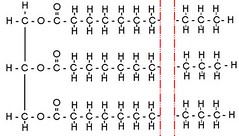
Glycerol + 3 Fatty Acids
When a glycerol molecule, C<sub>3</sub>H<sub>5</sub>(OH)<sub>3</sub> (the vertical part on the left of the image), picks up three fatty acids (the long strings of C and H on the right), they combine to form a triglyceride.
Triglycerides are fats and oils. If the long fatty acid chains all remain straight, each carbon bonding with two Hydrogen atoms and its two neighbor Carbon atoms, the triglyceride can pack densely, and thus becomes a solid at room temperature. This is a saturated fat.
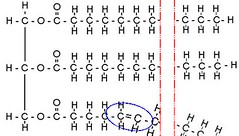
If one or more of the long fatty acids develops a "kink", ie two Carbons double bond and dump a Hydrogen, the stack can not pack as densely, and thus becomes a liquid at room temperature. This is an unsaturated oil. If there is one kink, it's a monounsaturated oil, and if more than one, it's a polyunsaturated oil.
Ta-da. It was kind of cool to suddenly understand the difference between them after having heard the terms for so long in reference to food labels.
We can measure energy in units called calories.
Because of fat's high percentage of hydrocarbons (all along those fatty acid chains), it has a high caloric content. For comparison, a gram of fat contains 9 calories, while a gram of carbohydrates contains 4 calories. Remember that those Hydrogen - Carbon bonds are high energy content because of the non-polar covalent bond.
Some properties of solid fats:
They are good insulators. (whales, walrus, Rush Limbaugh, etc. are good examples.)
They also make good shock absorbers. Vital organs are wrapped in fat, especially where not protected by bone.
3. Proteins
Life on earth is protein based.
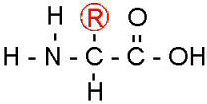
Proteins are macromolecules. They are polymers of amino acids.
On the left of this structural diagram of an amino acid is the amino functional group, and on the right, the carboxyl functional group. Note the Oxygen and the Hydroxide dangling off the end there. Those two really make an amino acid hydrophillic.
Note the big R in a circle. Checking the Periodic Table, you'll note that R is not an element. R is used as a place holder for whatever plug and play molecule might go there in any given amino acid. There are hundreds of different amino acids, depending on what gets plugged in there. Living things use twenty different amino acids to make proteins.
The R is what makes each of them different.
Amino Acids
There are three groups of amino acids.
Nine of them are nonpolar ---> They are made almost entirely of C and H
Six of them are polar.
Five of them are electrically charged ---> two are negatively charged, and three positively charged.
There's a nice amino acid chart on page 79 of our text book that shows these amino acids in their groups, and it will be important all semester. I won't bother to reproduce it here, though.

When two amino acids hook up, it's by a dehydration synthesis, similar to what we saw before in sugars. In this case though, we call it a peptide bond.
When two amino acids form a peptide bond, it's called a dipeptide. three is a tripeptide, and more than three is a polypeptide.
Long chains of amino acids, about when they reach 100 or so in a chain (not a hard and fast number), are called proteins. The average length of a protein in the human body is about 400 amino acids long.
There are three levels of protein structure that we looked at:
A) Primary Structure ---> proteins are a sequence of amino acids. A dipeptide can have 400 different possible primary structures (20 possible amino acids in the first position, 20 in the second = 20 X 20 = 400). A tripeptide can have 8,000 (20 X 20 X 20), a polypeptide of four amino acids 160,000 (20 X 20 X 20 X 20), etc. Remember, with proteins we're talking about at least about 100 amino acids long. The sequence of amino acids is critical at each and every location.
B) Secondary Structure ---> Hydrogen Bonding causes kinking, curling, twisting, etc, causing a protein to look like a twisted or knotted slinky.
C) Tertiary Structure ---> Complex 3D shape caused by the R groups interacting with each other and with the water solution in which they reside. The long molecules are electrically charged in places, polar in places and nonpolar in other places. The nonpolar parts are hydrophobic, the polar parts hydrophilic. So the nonpolar parts tend to wind up in the middle, while the polar parts and the fully charged parts tend to wind up around the outside, close to the water.
The end shape of a protein is determined by its primary structure and the Hydrogen Bonds, and the shape determines its function, much like the shape of a key determines which lock it will open.
Denaturation, the unwinding of the protein, is a result of broken Hydrogen bonds. Extreme heat or pH will denature a protein, and since shape determines function, a denatured protein will cease to function properly (or at all). High fever for instance (body temp of 42 or 43°) will denature the body's proteins and cause death. Death is bad.
Functions of Proteins
Body structure ---> Much of the body's structure is formed by proteins.
Contraction ---> Contraction of muscles (how you move) is a function of proteins
Enzymes ---> are proteins
Transport of molecules through the body
Hormones ---> insulin, growth hormone
Receptors ---> I apparently forgot to write down what Doc said about this.
These are just a few of the many functions of proteins.
4. Nucleic Acids
Macromolecules ---> Biggest of organic molecules
Polymers of nucleotides
pentose, phosphate group, nitrogenous base
Two types of Nucleic Acids ----> Ribonucleic Acid (RNA) and Deoxyribonucleic Acid (DNA)
We'll discuss these more later on, but here's where the lecture ended.
The following class period was our first exam, so there were no lecture notes for Wednesday, September 17<sup>th</sup>. We picked it up on Friday, September 19<sup>th</sup>.
--------------
“Why do creationists have such a hard time with commas?
Linky“. ~ Steve Story, Legend
|


















