Lou FCD

Posts: 5455
Joined: Jan. 2006
|

My notes and thoughts from Biology 111, for Wednesday, August 27, 2008. The entire series can be found here.
Wednesday's lecture began with a review of atomic structure, including a reminder that our e- * diagrams are 2D representations of 3D space.
Then we moved on to some more basic chemistry.
We focused mostly on electrons, and will continue to, as electrons are what determines reactivity of an atom, and reactivity is what's really vital to biology.
e- orbits are called e- shells or energy levels. Each e- orbital can hold up to 2 e-.
The first energy level has one orbital, because it's so small, and electrons, having all the same negative electrical charge, repel each other.
The second and third energy levels each contain 4 orbitals, each energy level then is capable of holding 8 e- (2 e- in each orbital).
Then doc talked about how electrons fill from the innermost energy level, out.
e- contain Potential Energy due to location or structure.
Potential Energy is energy stored up that can be used to do work. For instance, because our lecture room is on the second floor, I have more potential energy than the student just below me on the first floor. Should a hole open up under my seat, I would fall down, releasing that potential energy as kinetic energy. That energy would be doing work, like breaking the table below me, breaking my bones, or with a water wheel type contraption the energy released by my falling could be used to produce electricity.
Now, in order to get that potential energy, I had to walk up the steps, doing work, trading kinetic energy for potential energy. So to get energy out, I first had to put energy in.
Remembering that the potential energy in my body on the second floor is caused by my distance from the center of gravity of the planet, likewise the potential energy of an electron is caused by its distance from the nucleus of an atom (though the force involved here would be electromagnetism rather than gravity). The further from the nucleus an e- is, the more potential energy it has.
e- must be in an orbital. They don't free range within the atom, and they tend towards the lowest energy level in which they can squeeze. Changing orbitals requires a change in energy. Energy into an electron causes the electron to move into higher energy levels, and energy released from an electron causes the electron to move to lower energy levels. This is often denoted by ?E, pronounced "delta E".
Doc used the example of sugar. Where does the energy we get from sugar come from? Ultimately, the sun imparts the energy to the plant via the leaves. Sunlight strikes the leaves of the sugarcane plant causing an excitement of electrons, and the plant stores that energy as potential energy. When we eat the sugar, our bodies change that back into kinetic energy, giving us a sugar rush. Thus in the end, we are eating sunlight.
So before we go any further with the notes, let's take a look at the elements we're discussing. In biology, most of the elements we're going discuss are going to be found within the first 18 elements on the periodic table. Let's have the standard periodic table, with those elements highlighted. (The original table here is from the National Institute of Standards and Technology, NIST. The electron distribution table is from a scan of page 36 in our textbook. I have put the two images together to help visualize the sections of the periodic table that we'll be discussing.)
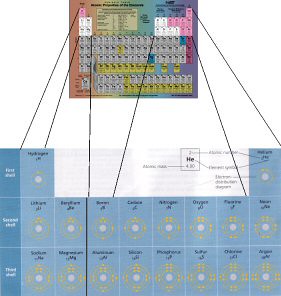
Next up, we talked about orbitals, arrangement of electrons in those orbitals at different energy levels, and the notation we use to describe all that. The remainder of this discussion will reference this electron distribution diagram, found on page 36 of our textbook (at my blog, you can click for a larger version, hosted at my Flickr account):
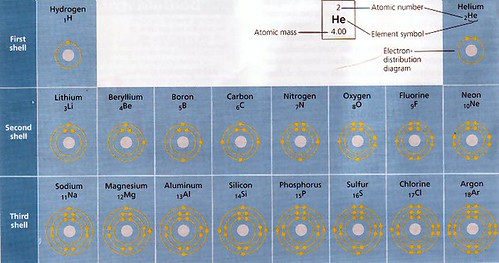
Let's look now at a few elements on this chart. The obvious place to start is at the beginning, so we'll start with Hydrogen.

In this image, I've highlighted Hydrogen (H). A hydrogen atom consists of one e- and one p+. There are no neutrons in the nucleus of a stable hydrogen atom. The single electron travels in the orbit described. For our purposes, we're going to ignore the nucleus for the time being. In this notation, we're mostly concerned with electrons and their orbits.
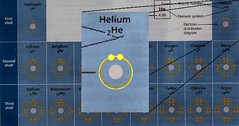
Comparing that to Helium (He), we see that in this notation, we're not going to separate the electrons on opposite sides of the atom as they physically would tend to be, we're just noting that there are two e- in this orbit. Atoms in the same row on the periodic table have the same number of shells, and in the first electron shell, there is one orbit. Each orbit will hold up to two e-. It's easy to think of this first shell as being so small that the charge of the e- (which repel each other) are just so close that they won't allow any more electrons in the vicinity.
Further out, in larger shells, each shell will consist of four orbits, but again, each orbit can only hold 2 e-. The outer e- shell of a given atom is referred to as the valence shell. The number of e- in the valence shell is of utmost importance to chemical reactions, and thus to biology.

In the second row then, we would expect to find two e- shells, and indeed, here is Lithium (Li). Notice that Li has the inner shell full (2 e-) and then begins to work on the next shell, with its third e-. Electrons always fill out the shells from the inside out, from closest to the nucleus, to furthest from the nucleus.

Remember that except for the innermost one, e- shells each contain four e- orbits, and each orbit holds two e-. Now, each of those orbits in a shell will take on e- before any of them will take a second e-. Thus the notation for Beryllium shows a second electron in the valence shell, but in a separate orbit from the first e-.

Boron (B) takes a third e-, in a third orbit of the second shell, and then we come to Carbon ( C ). Carbon has four valence e-, one in each orbit of the valence shell. Each of the four e- orbits of the valence shell now has a single electron.
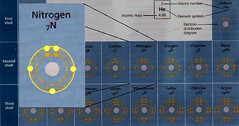
The next element, Nitrogen (N), has five valence e-, and the fifth one can now go in the first orbit of the valence shell, since each orbit in the shell now has one electron.

Predictably, Oxygen then takes a sixth e- in the valence shell (for a total of 8 e-) and it goes in the second orbit.

At this point a patterns should be emerging. Notice that in the first column, all the elements have one valence e-, in the second they all have two, etc.

In the eighth column, all the elements have 8 valence e- (except He), meaning that their valence shells are full. The elements in this column are referred to as the Noble Gases, or the Inert Gases. Elements with full valence shells are non-reactive. They are happy with the number of e- they have, and tend not to interact with other elements.
Atoms are most stable when they have NO unpaired electrons. Natural things tend toward their most stable state. A stack of bricks is less stable than those same bricks all flat on the ground. Likewise, elements tend towards having full valence shells.
We can say then that the reactivity of an atom depends on the number of unpaired valence e-. This becomes very important for biological chemistry.
Now that we had a good idea about valence e-, we could take that information and apply it.
Chemical Bonds
We began our discussion of chemical bonds with the first type, which is called Covalent Bonding.
There are a couple ways for atoms to find e- to fill up their valence shells. They can steal one from another atom (called ionic bonding, discussed in the next lecture). But like in life, bigger atoms are better at this than small atoms. To quote Doc directly,
| Quote | | "If you want to be successful at taking something from someone else, you had better be bigger than them, or they will whoop your ass and take it back. Hydrogen is the smallest atom, so it will always be the whoopee, not the whooper." |
So hydrogen goes a different route. It shares electrons. This is called a covalent bond.

In this fashion, two hydrogen atoms can each fill their valence shell (they each now have two e- in the valence shell). They are sharing a single pair of electrons, so this is a single bond.
We can write this in a number of ways.
There is the molecular formula
H2
The structural formula
H-H
and the Lewis Dot diagram
H:H
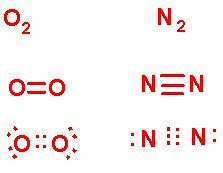
Oxygen, having two spots to fill in its valence shell, will share two pairs of electrons, and Nitrogen, 3. In the quick sketch I did above (forgive the lack of neatness there...), the molecular notation, the structural notation, and the Lewis Dot Diagram are given for Oxygen and Nitrogen, the two most abundant elements in the air we breathe.
The lecture ended there. On Friday, we discussed electronegativity. I'll get those notes up (hopefully) by tomorrow.
* remembering our shorthand, e- means electron, p+ is for proton, and n is for neutron.
Edited to clean up a butchered sentence.
Edited by Lou FCD on Aug. 30 2008,21:53
--------------
“Why do creationists have such a hard time with commas?
Linky“. ~ Steve Story, Legend
|


















