Lou FCD

Posts: 5455
Joined: Jan. 2006
|

My notes and thoughts from Biology 111, for Friday, August 29, 2008. The entire series can be found here.
Forgive the delay, but I've had a ton of stuff to work on.
On Friday, we started out with a review of covalent bonding. Doc re-stressed that in covalent bonding, atoms are sharing one or more pairs of electrons.
Let's take another look at our covalent bonding notation:
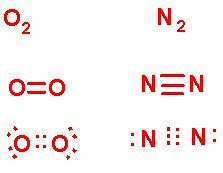
Now note that the two Oxygens share two pairs of e- and the two Nitrogens share three pairs of e-, as noted by the lines and by the dots between them. Also note that in the Lewis Dot diagram, all valence e- are depicted, regardless of whether they are involved in the bonding.
Let's throw some more elements together with covalent bonds:
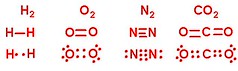
And to really point out the sharing, I've circled and colored to highlight which e- belong to which element:
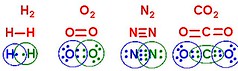
In this diagram, we can now really see that each element now has a full valence shell, by sharing e- part time with its neighbor.
Electronegativity
Electronegativity can be defined as the ability of an atom to attract e- to itself when in a compound.
Ignoring the Noble Gases (because they have full valence shells and don't tend to interact with other elements), we can roughly divide our Electron Distribution diagram into three parts. To the left side of the diagram we have elements that are very electropositive. It's much easier for them to lose a few e- to wind up with a full valence shell than to grab six or seven e- from somewhere else. In the center, elements are electroneutral, where they can sort of go either way. To the right of the diagram, are the electronegative elements, which only need an e- or two to fill their valence shell, and thus are more likely to take than to give.
This becomes important especially when we start discussing ionic bonds.
So if atoms are fairly close in terms of elelectronegativity, they will tend to share e- equally, and there is an even distribution of charge around the molecule. We call that a nonpolar colvalent bond.
If the atoms are further apart in terms of electronegativity, the more electronegative atom will tend to pull the shared e- more towards itself, and the distribution of charge around the molecule will have a very slight uneveness to it. We call that a polar covalent bond.
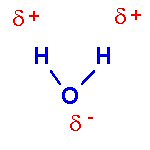
Water is a good example of a polar covalent bond. Because the Oxygen atom is highly electronegative and the Hydrogen atoms are very electropositive, the shared e- spend more time around the oxygen atom than the hydrogen atoms. Although the molecule maintains a net charge of zero (10 p+ and 10 e- in total), it does have a very slight negative charge on the Oxygen end and a very slight positive charge on the Hydrogen ends. That charge is denoted with the lower case Greek letter delta (?). Note that the atoms of a water molecule do not line up in a straight line like the atoms of a Carbon Dioxide molecule.
Ionic Bonds
Attraction between a cation and an anion.
Ionic bonds form when the difference in electronegativity between two atoms is great.

Sodium, for instance, is very electropositive, being way over on the left side of the diagram and having only one e- in its valence shell.

Chlorine is very electronegative, being way over on the right side of the diagram and having seven e- in its valence shell.
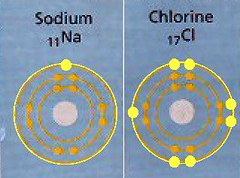
Sodium and Chlorine, side by side for a good look.
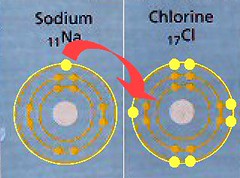
When they get together, Chlorine doesn't share an e- with Sodium, it steals one completely. This of course upsets the normal balance of an atom, where it usually has the same number of p+ and e-. When this happens, the atom becomes an ion. Because the Sodium ion has one fewer e- (10) than it has p+(11), it now has a net charge of +1, and it's called a cation. The Chlorine ion has one more e- (18) than it has p+(17) , so it has a net charge of -1 and is called an anion.
Remember that opposite charges attract each other. The Chlorine anion will now attract the Sodium cation, and they will form an ionic bond. This is not actually a molecule, but rather an ionic compound. Bonds only form molecules through covalent bonds.
Chapter 3
Water and the Fitness of the Environment
Water has several emergent properties that make it essential for life on Earth.
Hydrogen Bonds
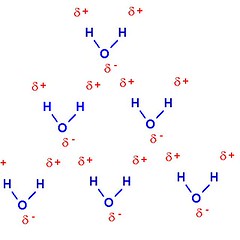
Water is a polar molecule, and can form Hydrogen Bonds. That very slight polarity we mentioned earlier now becomes very important, as we begin discussing how water molecules attract each other, with what's known as Hydrogen Bonding.
This is where the lecture left off, to be continued on Wednesday, 3 September.
Edited by Lou FCD on Sep. 02 2008,22:10
--------------
“Why do creationists have such a hard time with commas?
Linky“. ~ Steve Story, Legend
|


















